Services
- Home
- Services
Medical Device Product Engineering

We offer end-to-end new product development and project management expertise: taking a concept/prototype to design, development and production transfer with a focus on innovation and regulatory alignment. We can develop medTech systems spanning the following technical domains:
- Measurement sensors and catheters
- Product design/3D modeling
- Embedded device development
- Windows PC or mobile software development
- AI and cloud based solutions

Human Factor and Usability Evaluation
HFE is important not only to ensure safe medTech product, but also prevent product launch delays associated with late detection of user interface design shortcomings. Through systematic analysis of intended users, anticipated user, we help clients with high-quality and technically compliant usability evaluations.

Product Documentation
Our team has extensive experience in developing and maintaining the Design History File (DHF) as per US FDA 21 CFR 820.30 and ISO 13485:2016 for the project at hand.

Architecture and
Design
We employ balanced requirement and risk engineering principles to derive design inputs at system, subsystem and component level. Our multi-disciplinary engineering team is skilled in translating the design inputs into robust designs, taking reliability and manufacturability in consideration.

Design Transfer
Design transfer is the process of ensuring that device design is correctly translated into production specifications. To this end, we identify part suppliers, design production processes, and plan & conduct process validations.

Verification and Validation
A systematic V&V planning and execution ensures that the product performs as per the design input specifications and as per stakeholders needs. We utilize multi-tier testing methodologies for product verification – spanning software and hardware sub-systems and their integration into full system - to ensure thoroughness and requirement coverage.
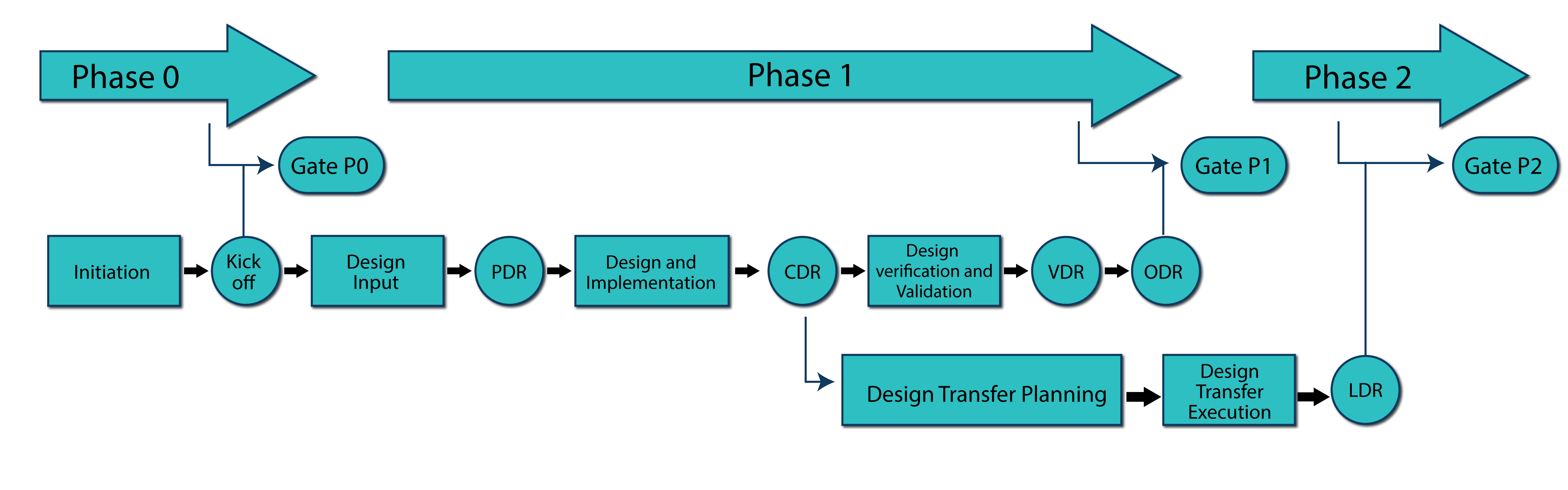
We follow strict phase-driven development framework so that both technical and business stakeholders have clear understanding of the expectations, the status and progress of the project. Design reviews are conducted at critical milestones, overseen by external independent technical experts. We have understanding of both waterfall and agile development methodologies, and we utilize adequate balance within our development framework.
